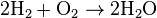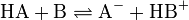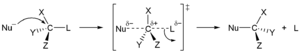A
thermite reaction using iron(III) oxide. The sparks flying outwards are globules of molten iron trailing smoke in their wake.
A
chemical reaction is a process that leads to the transformation of one set of
chemical substances to another.
Chemical reactions can be either
spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity. Classically, chemical reactions encompass changes that strictly involve the motion of
electrons in the forming and breaking of
chemical bonds, although the general concept of a chemical reaction, in particular the notion of a
chemical equation, is applicable to transformations of elementary particles (such as illustrated by
Feynman diagrams), as well as
nuclear reactions.
The substance (or substances) initially involved in a chemical reaction are called
reactants or reagents. Chemical reactions are usually characterized by a
chemical change, and they yield one or more
products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called
elementary reactions, and the information on the precise course of action is part of the
reaction mechanism. Chemical reactions are described with
chemical equations, which graphically present the starting materials, end products, and sometimes intermediate products and reaction conditions.
Different chemical reactions are used in combination in
chemical synthesis in order to obtain a desired product. In
biochemistry, series of chemical reactions
catalyzed by
enzymes form
metabolic pathways, by which syntheses and decompositions impossible under ordinary conditions are performed within a
cell.
Reaction types
Four basic types
Synthesis
In a synthesis reaction, two or more simple substances combine to form a more complex substance. Two or more reactants yielding one product is another way to identify a synthesis reaction. For example, simple hydrogen gas combined with simple oxygen gas can produce a more complex substance, such as water.
Decomposition
A decomposition reaction is the opposite of a synthesis reaction, where a more complex substance breaks down into its more simple parts.
Single replacement
In a single replacement reaction, a single uncombined element replaces another in a compound.
Double replacement
In a double replacement reaction, parts of two compounds switch places to form two new compounds.
This is when the anions and cations of two different molecules switch places, forming two entirely different compounds.
These reactions are in the general form:
AB + CD ---> AD + CB
One example of a double displacement reaction is the reaction of lead (II) nitrate with potassium iodide to form lead (II) iodide and potassium nitrate:
Pb(NO3)2 + 2 KI ---> PbI2 + 2 KNO3
Oxidation and reduction
Illustration of a redox reaction

The two parts of a redox reaction
Redox reactions can be understood in terms of transfer of electrons from one involved species (
reducing agent) to another (
oxidizing agent). In this process, the former species is oxidized and the latter is reduced, thus the term
redox. Though sufficient for many purposes, these descriptions are not precisely correct.
Oxidation is better defined as an increase in
oxidation number, and reduction as a decrease in oxidation number. In practice, the transfer of electrons will always change the oxidation number, but there are many reactions that are classed as "redox" even though no electron transfer occurs (such as those involving
covalent bonds).
An example of a redox reaction is:
- 2 S2O32−(aq) + I2(aq) → S4O62–(aq) + 2 I−(aq)
Here I
2 is reduced to I
– and S
2O
32– (
thiosulfate anion) is oxidized to S
4O
62–.
Which of the involved reactants would be reducing or oxidizing agent can be predicted from the
electronegativity of their elements. Elements with low electronegativity, such as most
metals, easily donate electrons and oxidize – they are reducing agents. On the contrary, many ions with high oxidation numbers, such as
H2O2,
MnO−
4,
CrO3,
Cr2O2−
7,
OsO4) can gain one or two extra electrons and are strong oxidizing agents.
The number of electrons donated or accepted in a redox reaction can be predicted from
electron configuration of the reactant element. Elements are trying to reach the low-energy
noble gas configuration, and therefore alkali metals and halogens will donate and accept one electron, respectively, and the noble gases themselves are chemically inactive.
An important class of redox reactions are the
electrochemical reactions, where the electrons from the power supply are used as a reducing agent. These reactions are particularly important for the production of chemical elements, such as
chlorine or
aluminium. The reverse process in which electrons are released in redox reactions and can be used as electrical energy is possible and is used in the batteries.
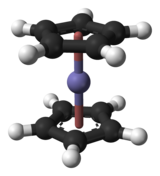
Complexation
In complexation reactions, several
ligands react with a metal atom to form a
coordination complex. This is achieved by providing
lone pairs of the ligand into empty
orbitals of the metal atom and forming
dipolar bonds. The ligands are
Lewis bases, they can be both ions and neutral molecules, such as carbon monoxide, ammonia or water. The number of ligands that react with a central metal atom can be found using the
18-electron rule, saying that the
valence shells of a
transition metal will collectively accommodate 18
electrons, whereas the symmetry of the resulting complex can be predicted with the
crystal field theory and
ligand field theory. Complexation reactions also include
ligand exchange, in which one or more ligands are replaced by another, and redox processes which change the oxidation state of the central metal atom.
[23]
Acid-base reactions
Acid-base reactions involve transfer of
protons from one molecule (
acid) to another (
base). Here,
acids act as proton donors and
bases as acceptors.
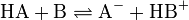
- Acid-base reaction, HA: acid, B: Base, A–: conjugated base, HB+: conjugated acid
The associated proton transfer results in the so-called
conjugate acid and conjugate base.
[24] The reverse reaction is possible, and thus the acid/base and conjugated base/acid are always in equilibrium. The equilibrium is determined by the
acid and base dissociation constants (
Ka and
Kb) of the involved substances. A special case of the acid-base reaction is the
neutralization where an acid and a base, taken at exactly same amounts, form a neutral
salt.
Acid-base reactions can have different definitions depending on the acid-base concept employed. Some of the most common are:
- Arrhenius definition: Acids dissociate in water releasing H3O+ ions; bases dissociate in water releasing OH– ions.
- Brønsted-Lowry definition: Acids are proton (H+) donors, bases are proton acceptors; this includes the Arrhenius definition.
- Lewis definition: Acids are electron-pair acceptors, bases are electron-pair donors; this includes the Brønsted-Lowry definition.
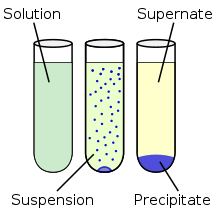
Precipitation
Precipitation is the formation of a solid in a solution or inside another solid during a chemical reaction. It usually takes place when the concentration of dissolved ions exceeds the
solubility limit
and forms an insoluble salt. This process can be assisted by adding a precipitating agent or by removal of the solvent. Rapid precipitation results in an
amorphous or microcrystalline residue and slow process can yield single
crystals. The latter can also be obtained by
recrystallization from microcrystalline salts.
Solid-state reactions
Reactions can take place between two solids. However, because of the relatively small
diffusion rates in solids, the corresponding chemical reactions are very slow. They are accelerated by increasing the reaction temperature and finely dividing the reactant to increase the contacting surface area.
Photochemical reactions
In
photochemical reactions, atoms and molecules absorb energy (
photons) of the illumination light and convert into an
excited state. They can then release this energy by breaking chemical bonds, thereby producing radicals. Photochemical reactions include hydrogen–oxygen reactions,
radical polymerization,
chain reactions and
rearrangement reactions.
Many important processes involve photochemistry. The premier example is
photosynthesis, in which most plants use solar energy to convert
carbon dioxide and water into
glucose, disposing of
oxygen as a side-product. Humans rely on photochemistry for the formation of vitamin D, and
vision is initiated by a photochemical reaction of
rhodopsin.
In
fireflies, an
enzyme in the abdomen catalyzes a reaction that results in
bioluminescence.
Many significant photochemical reactions, such as ozone formation, occur in the Earth atmosphere and constitute
atmospheric chemistry.
Catalysis

Schematic potential energy diagram showing the effect of a catalyst in an endothermic chemical reaction. The presence of a catalyst opens a different reaction pathway (in red) with a lower activation energy. The final result and the overall thermodynamics are the same.
Solid heterogeneous catalysts are plated on meshes in ceramic
catalytic converters in order to maximize their surface area. This exhaust converter is from a
Peugeot 106 S2 1100
In
catalysis, the reaction does not proceed directly, but through a third substance known as
catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself; however, it can be inhibited, deactivated or destroyed by secondary processes. Catalysts can be used in a different phase (
heterogeneous) or in the same phase (
homogenous) as the reactants. In heterogeneous catalysis, typical secondary processes include
coking where the catalyst becomes covered by
polymeric side products. Additionally, heterogeneous catalysts can dissolve into the solution in a solid–liquid system or evaporate in a solid–gas system. Catalysts can only speed up the reaction – chemicals that slow down the reaction are called inhibitors.
[30][31] Substances that increase the activity of catalysts are called promoters, and substances that deactivate catalysts are called catalytic poisons. With a catalyst, a reaction which is kinetically inhibited by a high activation energy can take place in circumvention of this activation energy.
Heterogeneous catalysts are usually solids, powdered in order to maximize their surface area. Of particular importance in heterogeneous catalysis are the
platinum group metals and other transition metals, which are used in
hydrogenations,
catalytic reforming and in the synthesis of commodity chemicals such as
nitric acid and
ammonia. Acids are an example of a homogeneous catalyst, they increase the nucleophilicity of
carbonyls, allowing a reaction that would not otherwise proceed with electrophiles. The advantage of homogeneous catalysts is the ease of mixing them with the reactants, but they may also be difficult to separate from the products. Therefore, heterogeneous catalysts are preferred in many industrial processes.
Reactions in organic chemistry
In organic chemistry, in addition to oxidation, reduction or acid-base reactions, a number of other reactions can take place which involve
covalent bonds between carbon atoms or carbon and
heteroatoms (such as oxygen, nitrogen,
halogens, etc.). Many specific reactions in organic chemistry are
name reactions designated after their discoverers.
Substitution
In a
substitution reaction, a
functional group in a particular
chemical compound is replaced by another group.
These reactions can be distinguished by the type of substituting species into a
nucleophilic,
electrophilic or
radical substitution.
In the first type, a
nucleophile, an atom or molecule with an excess of electrons and thus a negative charge or
partial charge, replaces another atom or part of the "substrate" molecule. The electron pair from the nucleophile attacks the substrate forming a new bond, while the
leaving group departs with an electron pair. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. Examples of nucleophiles are
hydroxide ion,
alkoxides,
amines and
halides. This type of reaction is found mainly in
aliphatic hydrocarbons, and rarely in
aromatic hydrocarbon. The latter have high electron density and enter
nucleophilic aromatic substitution only with very strong
electron withdrawing groups. Nucleophilic substitution can take place by two different mechanisms,
SN1 and
SN2. In their names, S stands for substitution, N for nucleophilic, and the number represents the
kinetic order of the reaction, unimolecular or bimolecular.
The three steps of an
SN2 reaction. The nucleophile is green and the leaving group is red
SN2 reaction causes stereo inversion (Walden inversion)
The S
N1 reaction proceeds in two steps. First, the
leaving group is eliminated creating a
carbocation. This is followed by a rapid reaction with the nucleophile.
In the S
N2 mechanism, the nucleophile forms a transition state with the attacked molecule, and only then the leaving group is cleaved. These two mechanisms differ in the
stereochemistry of the products. S
N1 leads to the non-stereospecific addition and does not result in a chiral center, but rather in a set of
geometric isomers (
cis/trans). In contrast, a reversal (
Walden inversion) of the previously existing stereochemistry is observed in the S
N2 mechanism.
Electrophilic substitution is the counterpart of the nucleophilic substitution in that the attacking atom or molecule, an
electrophile, has low electron density and thus a positive charge. Typical electrophiles are the carbon atom of
carbonyl groups, carbocations or
sulfur or
nitronium cations. This reaction takes place almost exclusively in aromatic hydrocarbons, where it is called
electrophilic aromatic substitution. The electrophile attack results in the so-called σ-complex, a transition state in which the aromatic system is abolished. Then, the leaving group, usually a proton, is split off and the aromaticity is restored. An alternative to aromatic substitution is electrophilic aliphatic substitution. It is similar to the nucleophilic aliphatic substitution and also has two major types, S
E1 and S
E2

Mechanism of electrophilic aromatic substitution
In the third type of substitution reaction, radical substitution, the attacking particle is a
radical.
This process usually takes the form of a
chain reaction, for example in the reaction of alkanes with halogens. In the first step, light or heat disintegrates the halogen-containing molecules producing the radicals. Then the reaction proceeds as an avalanche until two radicals meet and recombine.


- Reactions during the chain reaction of radical substitution
Addition and elimination
The
addition and its counterpart, the
elimination, are reactions which change the number of substituents on the carbon atom, and form or cleave
multiple bonds.
Double and
triple bonds can be produced by eliminating a suitable leaving group. Similar to the nucleophilic substitution, there are several possible reaction mechanisms which are named after the respective reaction order. In the E1 mechanism, the leaving group is ejected first, forming a carbocation. The next step, formation of the double bond, takes place with elimination of a proton (
deprotonation). The leaving order is reversed in the E1cb mechanism, that is the proton is split off first. This mechanism requires participation of a base.
Because of the similar conditions, both reactions in the E1 or E1cb elimination always compete with the S
N1 substitution.
 |
|  |
| E1 elimination |
| E1cb elimination |
The E2 mechanism also requires a base, but there the attack of the base and the elimination of the leaving group proceed simultaneously and produce no ionic intermediate. In contrast to the E1 eliminations, different stereochemical configurations are possible for the reaction product in the E2 mechanism, because the attack of the base preferentially occurs in the anti-position with respect to the leaving group. Because of the similar conditions and reagents, the E2 elimination is always in competition with the S
N2-substitution.

Electrophilic addition of hydrogen bromide
The counterpart of elimination is the addition where double or triple bonds are converted into single bonds. Similar to the substitution reactions, there are several types of additions distinguished by the type of the attacking particle. For example, in the
electrophilic addition of hydrogen bromide, an electrophile (proton) attacks the double bond forming a
carbocation, which then reacts with the nucleophile (bromine). The carbocation can be formed on either side of the double bond depending on the groups attached to its ends, and the preferred configuration can be predicted with the
Markovnikov's rule.
This rule states that "In the heterolytic addition of a polar molecule to an alkene or alkyne, the more electronegative (nucleophilic) atom (or part) of the polar molecule becomes attached to the carbon atom bearing the smaller number of hydrogen atoms."
If the addition of a functional group takes place at the less substituted carbon atom of the double bond, then the electrophilic substitution with acids is not possible. In this case, one has to use the
hydroboration–oxidation reaction, where in the first step, the
boron atom acts as electrophile and adds to the less substituted carbon atom. At the second step, the nucleophilic
hydroperoxide or halogen
anion attacks the boron atom.
While the addition to the electron-rich alkenes and alkynes is mainly electrophilic, the
nucleophilic addition plays an important role for the carbon-heteroatom multiple bonds, and especially its most important representative, the carbonyl group. This process is often associated with an elimination, so that after the reaction the carbonyl group is present again. It is therefore called addition-elimination reaction and may occur in carboxylic acid derivatives such as chlorides, esters or anhydrides. This reaction is often catalyzed by acids or bases, where the acids increase by the electrophilicity of the carbonyl group by binding to the oxygen atom, whereas the bases enhance the nucleophilicity of the attacking nucleophile.
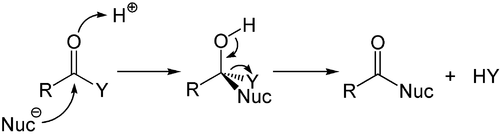
Acid-catalyzed addition-elimination mechanism
Nucleophilic addition of a
carbanion or another
nucleophile to the double bond of an
alpha, beta unsaturated carbonyl compound can proceed via the
Michael reaction, which belongs to the larger class of
conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds.
Some additions which can not be executed with nucleophiles and electrophiles, can be succeeded with free radicals. As with the free-radical substitution, the
radical addition proceeds as a chain reaction, and such reactions are the basis of the
free-radical polymerization.
Other organic reaction mechanisms

The Cope rearrangement of 3-methyl-1,5-hexadiene
Mechanism of a Diels-Alder reaction
Orbital overlap in a Diels-Alder reaction
In a
rearrangement reaction, the carbon skeleton of a
molecule is rearranged to give a
structural isomer of the original molecule. These include
hydride shift reactions such as the
Wagner-Meerwein rearrangement, where a
hydrogen,
alkyl or
aryl group migrates from one carbon to a neighboring carbon. Most rearrangements are associated with the breaking and formation of new carbon-carbon bonds. Other examples are
sigmatropic reaction such as the
Cope rearrangement.
Cyclic rearrangements include
cycloadditions and, more generally,
pericyclic reactions, wherein two or more double bond-containing molecules form a cyclic molecule. An important example of cycloaddition reaction is the
Diels–Alder reaction (the so-called [4+2] cycloaddition) between a conjugated
diene and a substituted
alkene to form a substituted
cyclohexene system.
Whether or not a certain cycloaddition would proceed depends on the electronic orbitals of the participating species, as only orbitals with the same sign of
wave function will overlap and interact constructively to form new bonds. Cycloaddition is usually assisted by light or heat. These perturbations result in different arrangement of electrons in the excited state of the involved molecules and therefore in different effects. For example, the [4+2] Diels-Alder reactions can be assisted by heat whereas the [2+2] cycloaddition is selectively induced by light.
Because of the orbital character, the potential for developing
stereoisomeric products upon cycloaddition is limited, as described by the
Woodward-Hoffmann rules.
Biochemical reactions
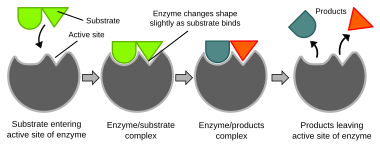
Illustration of the induced fit model of enzyme activity
Biochemical reactions are mainly controlled by
enzymes. These
proteins can specifically
catalyze a single reaction, so that reactions can be controlled very precisely. The reaction takes place in the
active site, a small part of the enzyme which is usually found in a cleft or pocket lined by
amino acid residues, and the rest of the enzyme is used mainly for stabilization. The catalytic action of enzymes relies on several mechanisms including the molecular shape ("induced fit"), bond strain, proximity and orientation of molecules relative to the enzyme, proton donation or withdrawal (acid/base catalysis), electrostatic interactions and many others.
The biochemical reactions that occur in living organisms are collectively known as
metabolism. Among the most important of its mechanisms is the
anabolism, in which different
DNA and enzyme-controlled processes result in the production of large molecules such as
proteins and
carbohydrates from smaller units.
Bioenergetics studies the sources of energy for such reactions. An important energy source is
glucose, which
can be produced by plants via
photosynthesis or assimilated from food. All organisms use this energy to produce
adenosine triphosphate (ATP), which can then be used to energize other reactions.
Applications

Thermite reaction proceeding in railway welding. Shortly after this, the liquid iron flows into the mould around the rail gap
Chemical reactions are central to
chemical engineering where they are used for the synthesis of new compounds from natural raw materials such as
petroleum and mineral
ores. It is essential to make the reaction as efficient as possible, maximizing the yield and minimizing the amount of reagents, energy inputs and waste.
Catalysts are especially helpful for reducing the energy required for the reaction and increasing its
reaction rate.
Some specific reactions have their niche applications. For example, the
thermite reaction is used to generate light and heat in
pyrotechnics and
welding. Although it is less controllable than the more conventional
oxy-fuel welding,
arc welding and
flash welding, it requires much less equipment and is still used to mend rails, especially in remote areas.
Monitoring
Mechanisms of monitoring chemical reactions depend strongly on the reaction rate. Relatively slow processes can be analyzed in situ for the concentrations and identities of the individual ingredients. Important tools of real time analysis are the measurement of
pH and analysis of optical absorption (color) and emission spectra. A less accessible but rather efficient method is introduction of a radioactive isotope into the reaction and monitoring how it changes over time and where it moves to; this method is often used to analyze redistribution of substances in the human body. Faster reactions are usually studied with
ultrafast laser spectroscopy where utilization of
femtosecond lasers allows short-lived transition states to be monitored at time scaled down to a few femtoseconds.
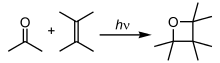
 products, as shown in the following example,
products, as shown in the following example,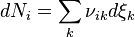 .
. .
.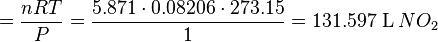
 and
and